The diseases - leishmaniasis and trypanosomiasis
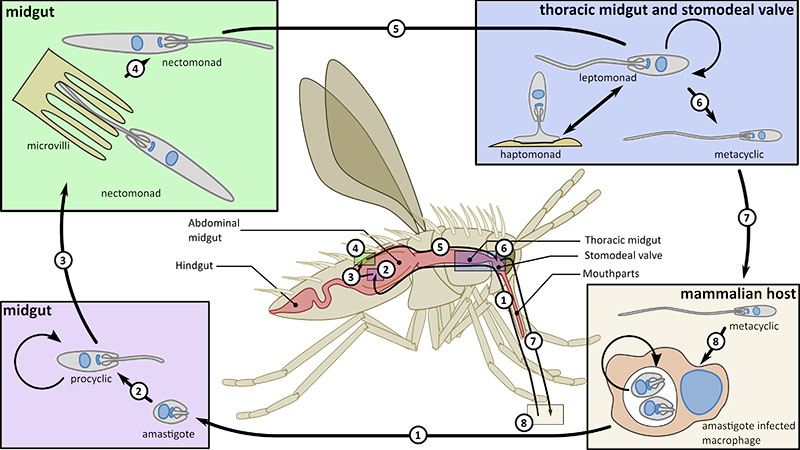
Both Leishmania and trypanosomes have a complex life cycle in which they are transmitted by an insect vector between mammalian hosts. Leishmania causes a range of different diseases from the severe and fatal visceral leishmaniasis to the self-healing cutaneous leishmaniasis and is found throughout the tropics and into the southern Mediterranean. Trypanosoma brucei on the other hand is restricted to sub-Saharan Africa, where it causes the fatal disease African sleeping sickness.
Research projects
Assembly of cell-to-cell and cell-to-substrate attachment structures
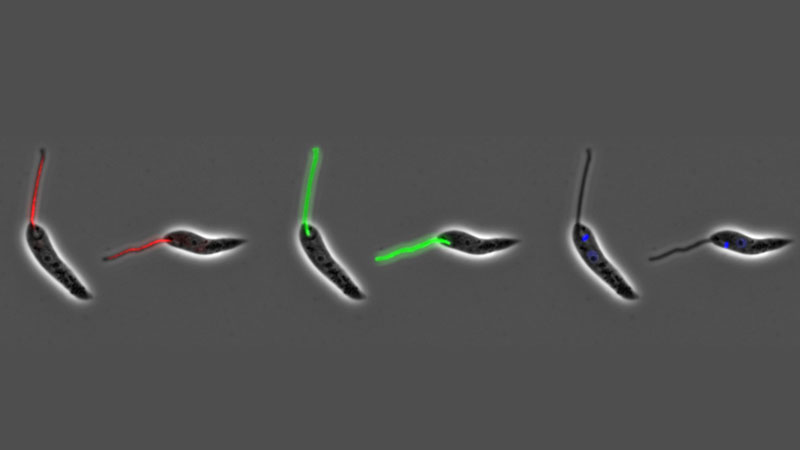
The attachment of cells to either other cells or substrates is a fundamental attribute of eukaryotic cells, both microbial and metazoan, and is central to the development of multicellularity. Microbes in a ‘free-living’ environment attach to substrates, often in complex biofilms, to maintain themselves within a specific ecological niche. However, attachment is also central to parasitism and the trypanosome and Leishmania parasites are excellent systems to study attachment as they form both cell:cell and cell:substrate transmembrane attachment structures. They have a cell:cell junctional structure called the Flagellum Attachment Zone (FAZ) that connects the flagellum to the cell body. This structure is crucial for defining cell shape and form; we have defined its architecture and using innovative proteomic approaches, we discovered 8 proteins in the FAZ and showed that FAZ is assembled at a spatially distinct site to that of the flagellum. In addition, these parasites utilise their flagella to attach to substrates in their insect vectors, where they form an attachment plaque - a cell:substrate transmembrane attachment structure. Recently, we have begun to investigate the architecture and assembly of the attachment plaque in trypanosomes and Leishmania.
Key questions:
- How is the assembly of architecturally complex structures outside of the cell co-ordinated?
- How are existing structures such as a motile flagellum repurposed for attachment functions?
Example papers:
- Alves AA et al., (2020) Control of assembly of extra-axonemal structures: the paraflagellar rod of trypanosomes. J Cell Sci. 133(10):jcs242271.
- Sunter JD et al., (2015) Flagellum attachment zone protein modulation and regulation of cell shape in Trypanosoma brucei life cycle transitions. J Cell Sci. 128, 3117-3130. PMC4541047.
Architecture of host-parasite interfaces
The flagellar pocket, present in microbes to mammalian cells, is a specialised region of the cell membrane at the base of the cilium/flagellum. In the trypanosome and Leishmania parasites it is the only site of endocytosis and exocytosis as well as the site of component sorting for flagellum construction. In concert with collaborators, we defined the detailed 3D structure of the Leishmania flagellar pocket and showed that it is remodelled during the life cycle, demonstrating that flagellar pocket architecture responds to changes in the extracellular environment and its functional requirements. In Leishmania we have shown that deletion of a single FAZ protein, FAZ5, alters flagellar pocket architecture and has a dramatic impact on parasite survival in vivo, illustrating the importance of understanding how cellular architecture is designed for pathogenicity. We have continued this work by undertaking a systematic screen of the role of FAZ proteins in flagellar pocket morphogenesis and function in Leishmania.
Key questions:
- How does flagellar pocket architecture contribute to its endocytic and associated functions?
- How does flagellar pocket architecture influence infectivity in the host/vector?
Example papers:
- Halliday C et al., (2020) Role for the flagellum attachment zone in Leishmania anterior cell tip morphogenesis. PLoS Pathog. 16(10):e1008494.
- Sunter JD et al., (2019) Leishmania flagellum attachment zone is critical for flagellar pocket shape, development in the sand fly, and pathogenicity in the host. Proc Natl Acad Sci U S A. 116:6351-6360.